ASCO Genitourinary Cancers Symposium
First Author: Lisa Dwyer Orr, MPH
Abstract Number: 229
Poster Board Number: G2
Session Information: 2/13/2025, 11:25 AM-12:45 PM PST
Poster Transcript
Background: Clinical trials are the foundation of oncologic drug development. Historically, only 23% of oncology trials are conducted in the community despite >70% of patients being treated this setting, leading to a disconnect between the therapy being tested and target population. Also, patients are hesitant to enroll in trials- in 2020, only 49% were willing, down from 85% in 2019. One way to improve this is participation by community oncologists, allowing patients to stay close to home. Targeted therapies are increasingly being approved by the FDA, with 89 small molecule drugs approved by 2020. Given the above landscape, Precision Health Informatics (PHI) collaborated with Mirati Therapeutics to pilot a clinical trial enrichment program focused on increasing enrollment of patients in the community related to efficacy and outcomes of adagrasib (KRAS G12C inhibitor) for NSCLC and CRC.
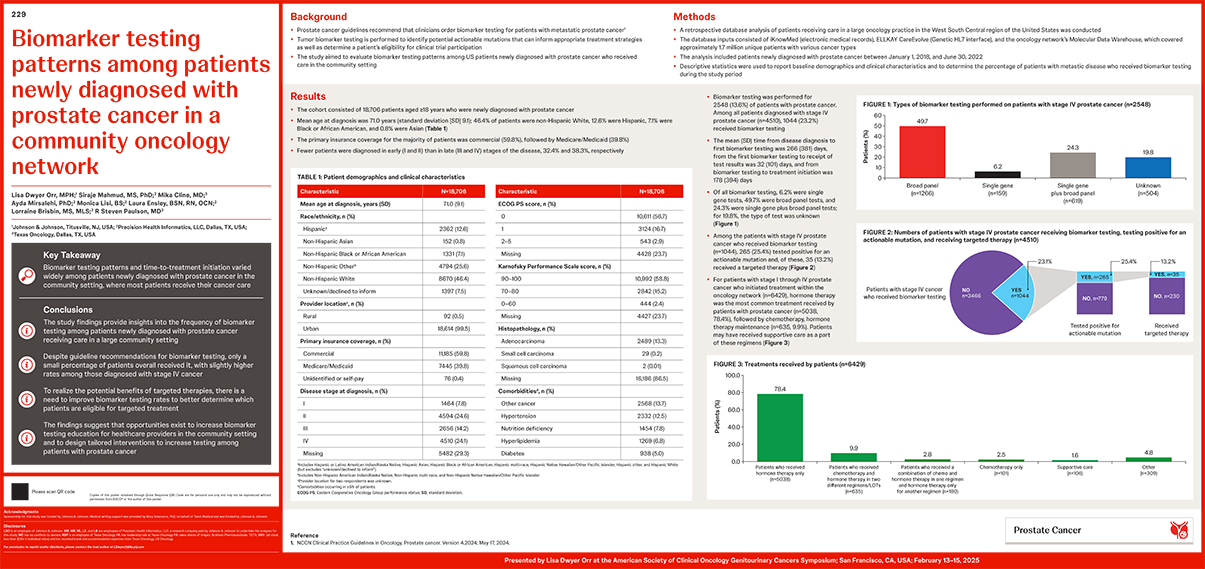
Methods: A retrospective database analysis, using several sources that include iKnowMed(EMR), EllkayCareEvolve (GeneticHL7interface), and the Molecular Data Warehouse and covers approximately 1.7 million distinct patients, was conducted on patients newly diagnosed with prostate cancer between Jan 1, 2018, and Jun 30, 2022, and receiving care in a large oncology network in West South-Central United States. Baseline demographics and clinical characteristics were analyzed descriptively and the proportion of patients who received biomarker testing was determined.
Results: Data on 18,743 patients with prostate cancer (mean age at diagnosis, 71 years; 46% White, 13% Hispanic, 7% Black, 1% Asian, 33% other/unknown) were abstracted. The proportion of patients who received biomarker testing was 14% overall and 23% among those with Stage IV disease. Of all biomarker tests performed, roughly 6% was single gene testing, 49% was broad panel testing, 25% was both single gene testing with broad panel testing, and approximately 20% of the tests were unknown. The average time from first biomarker testing to disease diagnosis was approximately 5 months. Less than 10% of the patients who tested positive with an actionable mutation received targeted therapy. Hormonetherapy (32%) was the most common treatment received, followed by supportive care (7%).
Conclusions: These findings provide insights into the frequency of biomarker testing among patients newly diagnosed with prostate cancer who receive care in a large community practice setting. Advancement in targeted therapies is predicated on improved testing rates, and this study suggests opportunities exist for interventions and increased education among healthcare providers based on observed time to testing and the rate of testing.