ASCO Annual Meeting, 2025
First Author: Donald Richards
Abstract Number: 3580
Poster Board Number: 249
Session Information: 5/31/2025, 9:00 AM-12:00 PM CT
Poster Transcript
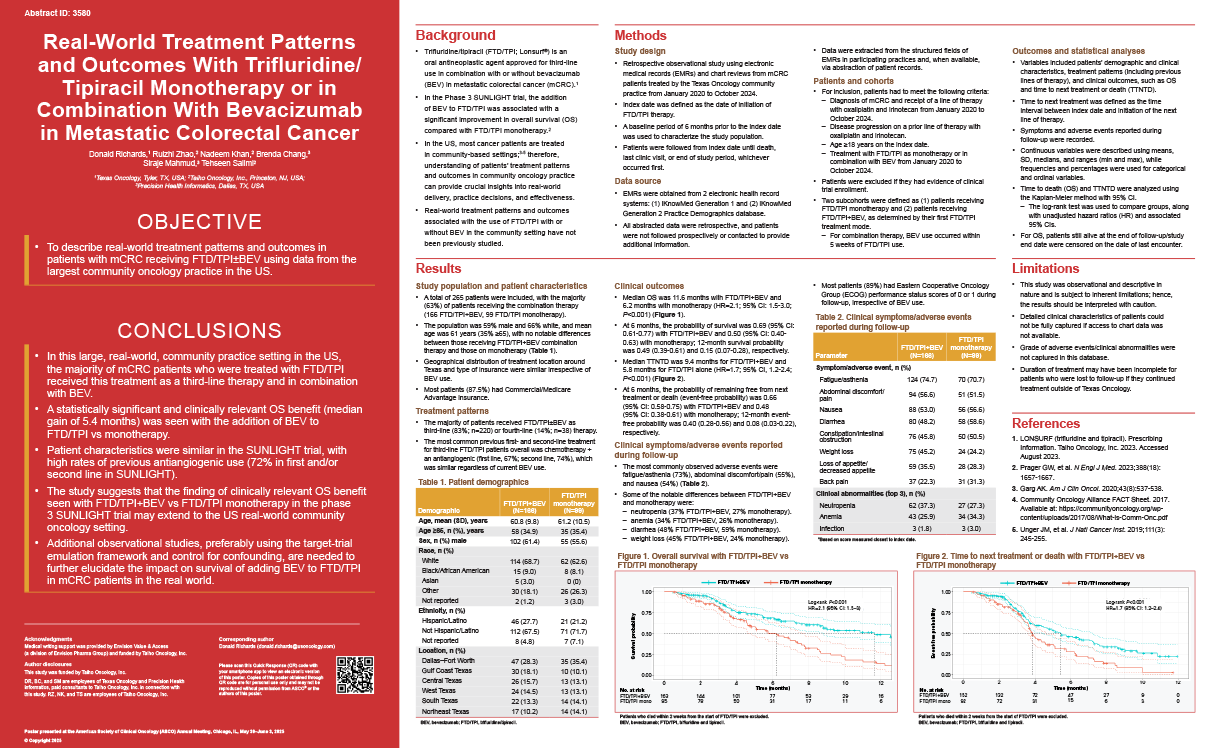
Background: Trifluridine/tipiracil (FTD-TPI; Lonsurf) is an oral antineoplastic agent approved for 3rd-line use in combination with or without bevacizumab (BEV) in metastatic colorectal cancer (mCRC). In the Phase III SUNLIGHT trial, the addition of BEV to FTD-TPI was associated with a significant improvement in overall survival (OS) and progression-free survival (PFS) compared to FTD-TPI monotherapy. However, data on the use of FTD-TPI in combination with BEV in the real-world community setting are currently limited.
Methods: This was a retrospective observational study involving electronic medical records and (where available) chart reviews from mCRC patients treated by the Texas Oncology community practice from Jan 2020 to Oct 2024. Patients had to have received FTD-TPI with or without BEV after progressing on a prior line of therapy with oxaliplatin and irinotecan. Variables included patient characteristics, clinical characteristics, treatment patterns and clinical outcomes. OS and time to next treatment or death (TTNTD) were analyzed using the Kaplan-Meier method.
Results: In total, 265 patients were included (166 FTD-TPI + BEV; 99 FTD-TPI monotherapy), with the majority receiving FTD-TPI as 3rd-line (83%; n = 220) or 4th-line (14%; n = 38) therapy. The population was 59% male, 66% white, and 35% were ≥65 years of age. The most common previous 1st- and 2nd-line treatment for 3rd-line FTD-TPI patients was chemotherapy + an antiangiogenic (1st-line, 67%; 2nd-line, 74%), which was similar regardless of current BEV use. Median duration of therapy was 2.8 months (range 0.3 to 12.5) with FTD-TPI + BEV and 2.8 months (range 0.1 to 10.4) with monotherapy. Median OS was 11.6 months with FTD-TPI + BEV and 6.2 months with monotherapy (hazard ratio [HR] = 2.1; 95% confidence interval [CI]: 1.5-3.0; p < 0.001). At 6 months, OS probability was 0.69 (95% CI: 0.61-0.77) with FTD-TPI + BEV and 0.50 (95% CI: 0.40-0.63) with monotherapy; 12-month OS probability was 0.49 (0.39-0.61) and 0.15 (0.07-0.28), respectively. Median TTNTD was 9.4 months for FTD-TPI + BEV and 5.8 months for FTD-TPI alone (HR = 1.7, 95% CI: 1.2-2.4; p < 0.001). The safety/tolerability profile was generally similar irrespective of BEV use, with the most common adverse events being fatigue/asthenia (73%), abdominal discomfort/pain (55%), and nausea (54%). The most notable difference was neutropenia (37% FTD-TPI + BEV, 27% monotherapy).
Conclusions: In this large real-world community practice setting in the US, FTD-TPI use in mCRC was mostly in the 3rd-line setting and approximately two-thirds of use was in combination with BEV. Patient characteristics were similar to the SUNLIGHT trial, with high rates of previous antiangiogenic use. A statistically significant and clinically relevant OS benefit was seen with the addition of BEV versus monotherapy consistent with the results of the SUNLIGHT trial.