2025 ASCO Quality Care Symposium
First Author: Shikha Prakash
Abstract Number: 505
Poster Board Number: P8
Session Information: 10/07/2025
ASCO Publication: JCO Oncol Pract 21, 505(2025) | Volume 21, Number 10_suppl | DOI: 10.1200/OP.2025.21.10_suppl.505
Poster Transcript
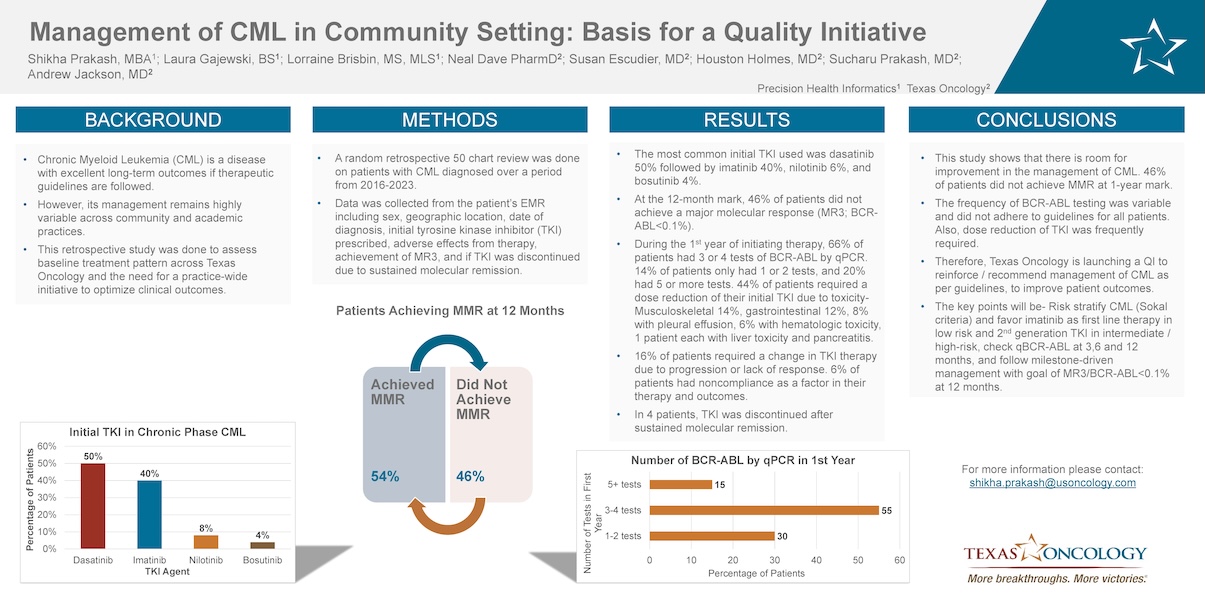
Background: Chronic Myeloid Leukemia (CML) is a disease with excellent long-term outcomes if therapeutic guidelines are followed. However, its management remains highly variable across community and academic practices. This retrospective study was done to assess baseline treatment pattern across Texas Oncology and the need for a practice-wide initiative to optimize clinical outcomes.
Methods: A random retrospective 50 chart review was done on patients in Texas Oncology with CML diagnosed over a period from 2016-2023. Data was collected from the patient’s electronic medical record including sex, geographic location, date of diagnosis, initial tyrosine kinase inhibitor (TKI) prescribed, adverse effects from therapy, achievement of MR3 (BCR-ABL transcripts ≤ 0.1%) and if TKI was discontinued due to sustained molecular remission.
Results: The most common initial TKI used was dasatinib (50%) followed by imatinib (40%), nilotinib 6%, and bosutinib 4%. At the 12-month mark, 46% of patients did not achieve a major molecular response (MR3; BCR-ABL<0.1%). During the first year of initiating therapy, 66% of patients had 3 or 4 tests of BCR-ABL by qPCR. 14% of patients only had 1 or 2 tests, and 20% had 5 or more tests. 44% of patients required a dose reduction of their initial TKI due to toxicity- Musculoskeletal (14%), gastrointestinal (12%), 8% with pleural effusion, 6% with hematologic toxicity, 1 patient each with liver toxicity and pancreatitis. 16% of patients required a change in TKI therapy due to progression or lack of response. 6% of patients had noncompliance as a factor in their therapy and outcomes. In 4 patients, TKI was discontinued after sustained molecular remission.
Conclusions: This study shows that there is room for improvement in the management of CML. 46% of patients did not achieve MMR at 1-year mark. Furthermore, the frequency of BCR-ABL testing was variable and did not adhere to guidelines for all patients. Also, dose reduction of TKI was frequently required. Therefore, Texas Oncology is launching a Quality Initiative to reinforce/recommend management of CML as per guidelines, to improve patient outcomes. This QI will recommend that all newly diagnosed patients with chronic phase CML be treated according to guidelines set forth by a “Hematology Committee”. The key points will be- Risk stratify CML (Sokal criteria) and favor imatinib as first line therapy in low risk and 2nd generation TKI in intermediate/high risk, check qBCR-ABL at 3,6 and 12 months, and follow milestone-driven management with goal of MR3/BCR-ABL<0.1% at 12 months.